What Term Best Describes a Reaction That Releases Energy
Today were doing problem for which of the following reactions releases energy and were given for choices because this is a multiple choice practice question for the AP biology exam. It is exothermic and has a negative AH.
What Is Difference Between Endothermic And Exothermic Reaction If Both Require Activation Energy Quora
4 What energy is released in chemical reactions.
. This means energy is released as the reaction occurs. The following describes an exergonic reaction - The reaction releases energy that is available to do work. Up to 24 cash back Set 1 Heats of Reaction Which statement best describes a chemical reaction in which energy is released.
2 It is exothermic and has a positive AH. An exergonic reaction is a reaction that releases free energy. The free energy of the system decreases.
An exergonic reaction may be called a spontaneous reaction or a favorable reaction. We first have to. The chemical bonds formed from the reaction are stronger than those that were broken in the reactants.
In chemical thermodynamics a chemical reaction where the change in the free energy is negative. What is the term for the amount of energy that needs to be added for a chemical reaction to start. An example of an exothermic reaction is the chemical reaction between sodium and chlorine which results in the formation of sodium chloride also known as common salt.
The relative potential energy of the reaction is negative. An endergonic reaction requires an input of free energy. Chemical reaction that release energy.
Exergonic reactions release energy to the surroundings. All chemical reactions that release energy in the form of heat are called exothermic reactions. Because this type of reaction releases energy rather than consuming it it can occur spontaneously without being forced by outside factors.
5 What is meant by the term activation energy. An exergonic reaction releases energy. In an exothermic reaction energy is released because the total energy of the products is less than the total energy of the reactants.
I understand what the basic energy source for all biological processes and that is probably heard of it. An exothermic reaction As an exothermic reaction occurs the energy of the reactants is higher than that of the products. Based on Reference Table I which change.
1 What Is The Term Used To Describe The Energy Needed To Get A Reaction Started. 3 It is endothermic and has a negative AH. Decreases the activation energy.
The energy released is called enthalpyA reaction that releases energy is called an exothermic reaction. What describes an exothermic reaction. The activation energy of the reaction is positive.
A substance that affects the reaction rate without being used up in the reaction Decomposition A reaction in which a compound breaks down into two or more simpler substances. Free energy is energy available to do work in an organism. BEndergonic reactions consume energy and exergonic reactions release energy.
In a chemical reaction such as the burning of paper energy is released as heat. The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. Okay so its understand this question.
Potential energy stored in the bonds of molecules - energy contained in an object because of its position or internal state - energy that is doing work - all potential available in a system active the substrate-binding site of an enzyme is known as the _____________ site. Releases more energy than it absorbs. The general term for a chemical reaction that releases heat is an exothermic reaction.
3 What is the term for the amount of energy that needs to be added for a chemical reaction to start quizlet. An exothermic reaction occurs when energy is released during a chemical reaction often times when chemical bonds are made. This energy is usually lost as heat energy or radiated as light.
Energy that drives the attachment of a nucleotide to the end of a growing st 000 What reaction will release the largest amount of energy to help power anothe. Exothermic reactions are reactions or processes that release energy usually in the form of heat or light. 2 What term is used to describe the energy required to start a reaction.
DEndergonic reactions take place slowly and exergonic reactions take place quickly. CBoth endergonic and exergonic reactions require a small amount of energy to overcome an activation barrier. Which of the following terms describes a reaction in which there is a net transfer of energy from a system to its surroundings - that is where more energy is released by bond formation than is consumed by bond cleavage.
Exergonic reactions are also called spontaneous reactions because they can occur without the addition of energy. Which phrase best describes the effect of a catalyst on a chemical reaction. In chemistry terms exergonic reactions are reactions where the change in free energy is negative.
4 It is endothermic and has a positive AH. Entropy is related to the constant input and release of energy which tends to increase disorder in the universe. OOOO the energy a compound possesses by virtue of its chemical bonds and their orientation the energy required to remove an electron from a gaseous atom the amount of energy absorbed or released when a chemical reaction takes place the energy required to separate the ions in a crystalline solid the minimum net kinetic energy that colliding particles must possess in order.
Which statement best describes the graph shown. What does it mean for a reaction to release energy1 point The relative potential energy of the reaction is positive. The relative potential energy of the reaction is positive.
The activation energy of the reaction is positive. What term is used to describe the energy change in this reaction.

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy
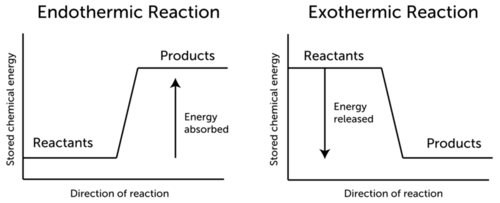
Conservation Of Energy In Chemical Reactions Read Chemistry Ck 12 Foundation
0 Response to "What Term Best Describes a Reaction That Releases Energy"
Post a Comment